Dementia Drug Development
Clinical research is medical research that involves people and that helps medical professionals to learn more about dementia and improve health care in the future. Clinical trials are part of clinical research with the goal to determine if treatment, prevention, and behavior approaches are safe and effective.
People may experience dementia differently which is why clinical research needs to include people with a variety of lived experiences and living conditions, as well as characteristics like race and ethnicity, age, sex, and sexual orientation, so that all communities benefit from scientific advances.
Before a human medicine receives a marketing authorization all clinical trial data is being reviewed and assessed by regional authorities. In Europe, this is the Committee for Medicinal Products for Humans Use (CHMP) of the European Medicine Agency (EMA) and in the United States this is the Food and Drug Administration (FDA).
It is not always common to talk about participating in clinical research after a dementia diagnosis and many people are unaware of clinical trial opportunities.
Here is where BLC can help!
See below Kay’s story and why she benefits from participation in a clinical trial.
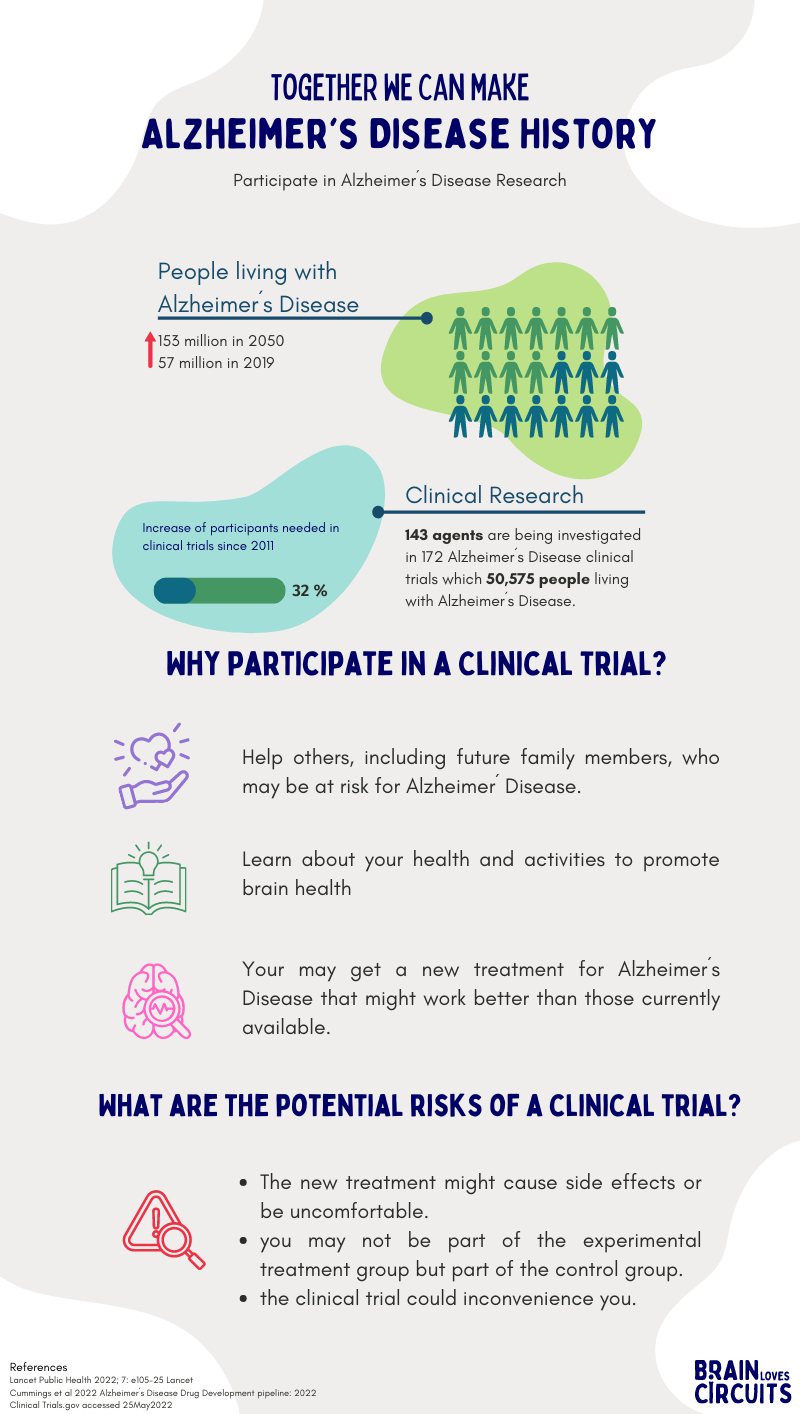
The following infographic provides a short summary of the Clinical Research in Alzheimer's Disease and please read the following article from the BLC founder Sabine and her colleagues about challenges in Neuroscience clinical trials.
Click here if you want more detailed information on clinical trials and the Alzheimer´s Disease drug development pipeline.
Dementia drug development is complex and very challenging and we are not able to capture all the information in great detail. Please contact us if you have any specific questions.